Biological Nitrification Process in Waste Water Treatment System
Definition
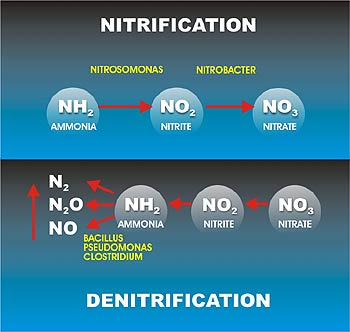
The removal of nitrogen by biological nitrification and denitrification is a two-step process. In the first step (nitrification), ammonia is converted aerobically to nitrate (NO3−). In the second step (denitrification), nitrates are converted to N2O or nitrogen gas (N2) under anoxic conditions. Two‐step biological process in which ammonia (NH4‐N) is oxidized to nitrite (NO2) and nitrite is oxidized to nitrate (NO3‐N).
Purpose of Nitrification
- Effect of ammonia on receiving water with respect to DO concentrations and fish toxicity
- Need to provide nitrogen removal to control eutrophication
- Need to provide nitrogen control for water‐reuse applications including groundwater recharge
- Drinking water maximum MCL for nitrate nitrogen is 45 mg/L as nitrate or 10 mg/L as nitrogen
- Total concentration of organic and ammonia nitrogen in municipal wastewater in the range 25‐ 45 mg/L as nitrogen based on flowrate of 450 L/capita.d (120 gal/capita.d)
- With limited water supplies, total nitrogen in excess of 200 mg/L as N measured in domestic wastewater
Nitrification Process
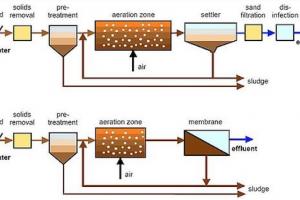
Nitrification process in waste water treatment is accomplished in both suspended growth and attached growth biological processes
Suspended Growth Processes
Nitrification along with BOD removal in single‐sludge process can be achieved, consisting of aeration tank, clarifier, and sludge recycle system
In case of toxic and inhibitory substances in wastewater, two‐sludge suspended growth system may be considered, consisting of two aeration tanks and two clarifiers in series. The first aeration tank/clarifier unit operated at short SRT for BOD and toxic substances removal, followed by nitrification in second aeration tank/clarifier unit operated at long SRT; Nitrifying bacteria grow much more slowly than heterotrophic bacteria.
Attached Growth Processes
- For nitrification, most of BOD must be removed before nitrifying organisms can be established
- Heterotrophic bacteria higher biomass yield and dominate surface area of fixed‐film systems over nitrifying bacteria;
- Nitrification accomplished in attached growth reactor after BOD removal or in separate attached growth system designed for nitrification.
- The nitrification rate for the attached-growth processes is higher than for the suspended-growth processes. Attached-growth processes normally carry more suspended solids in the effluent than the suspended-growth processes.
Microbiology of Nitrification
- Aerobic autotrophic bacteria are responsible for nitrification in activated sludge and biofilm processes;
- Two‐step process in nitrication involve two groups of bacteria; First stage, ammonia is oxidized to nitrite by one group (Nitrosomonas) and second stage, nitrite is oxidized to nitrate by another group of autotrophic bacteria (Nitrobacter)
- Other autotrophic bacteria for oxidation of ammonia to nitrite (prefix with Nitroso‐): Nitrosococcus, Nitrosospira, Nitrosolobus, and Nitrosorobrio
- Other autotrophic bacteria for oxidation of nitrite to nitrate (prefix with Nitro‐): Nitrococcus, Nitrospira, Nitrospina, and Nitroeystis
Factors affecting Process of Nitrification
Environmental Factors: pH
- Nitrification process in waste water treatment is pH sensitive and rates decline significantly at pH values below 6.8; Optimal nitrification rates occur at pH values in 7.5‐8.0 range; pH of 7.0 to 7.2 is normally used;
- Low alkaline waters require alkalinity to be added to maintain acceptable pH values;
- Amount of alkalinity added depends on initial alkalinity concentration and amount of NH4‐N to be oxidized;
- Alkalinity added in form of lime, soda ash, sodium bicarbonate, or magnesium hydroxide.
Environmental Factors: Toxicity
- Nitrifiers are good indicators of presence of organic toxic compounds at low concentrations;
- Toxic compounds include: Solvent organic chemicals, amines, proteins, tannins, phenolic compounds, alcohols, cyanates, ethers, carbamates, and benzene
Environmental Factors: Metals
- Complete inhibition of ammonia oxidation at 0.25 mg/L nickel, 0.25 mg/L chromium, and 0.10 mg/L copper
- Environmental Factors: Un‐ionized Ammonia
- Nitrification is also inhibited by un‐ionized ammonia (NH3) or free ammonia, and un‐ionized nitrous acid (HNO2);
- Inhibition effects are dependent on total nitrogen species concentration, temperature, and pH.